What is Allicin?
Table of Contents
What is Allicin?
Garlic cloves have a strong pungent smell and flavor, which comes from a compound called allicin. Allicin (Diallyl thiosulfinate) is produced by garlic cloves as a defense mechanism against pest attacks. On crushing or chopping, alliin (an odorless compound also known as S-allyl-L-cysteine sulfoxide) present in garlic cloves reacts with an enzyme called alliinase to produce allicin. Cavallito and Bailey were the first to discover allicin in 1944. S-alk(en)yl-l-cysteine sulfoxides are present in the family members of Alliaceae, including garlic, onion, Brassica genus, and rucola of Eruca genus, among other members of Brassicaceae. S-alk(en)yl-l-cysteine sulfoxides are responsible for the strong pungent odor of garlic, onion, shallots, etc. Of all the thiosulfinates found in garlic, more than two-thirds of the amount is allicin. About 5 mg of allicin is found in every clove (10 g) of garlic.
2.2K views
Two types of onions and two types of garlic as sources of allicin

Allicin's Chemical Formula
Allicin is an organosulfur compound, which means it is organic and contains sulfur. Organosulfur compounds are known for their characteristic odor and are widely present in the environment — they can be found in oceans, volcanoes, living beings on earth, and even in interstellar space. These compounds can be found in many hormones, proteins, vitamins, tripeptide glutathione, enzymes, and coenzymes. Thus, they can be obtained from both plants and animals. Some foods containing the organosulfur compounds include cabbage, cauliflower, garlic, onion, meat, eggs, and fish. They are further classified on the basis of the functional groups to which they are attached. The two main classes of organosulfur compounds in intact garlic are gamma glutamyl-S-allyl-L-cysteine and S-allyl-L-cysteine sulfoxide (alliin). These are volatile compounds and quickly decompose.
As mentioned earlier, allicin is a thiosulfinate; its chemical name is S-allyl prop-2-ene-1-sulfinothioate. The standard name given to allicin by the International Union of Pure and Applied Chemistry (IUPAC) is 3-prop-2-enylsulfinylsulfanylprop-1-ene. The chemical formula of allicin is C6H10OS2. Thus, it contains 6 carbon atoms, 10 hydrogen atoms, 2 sulfur atoms, and 1 oxygen atom.
Structure of Allicin
Synthesis of allicin from alliin via condensation of sulfenic acids
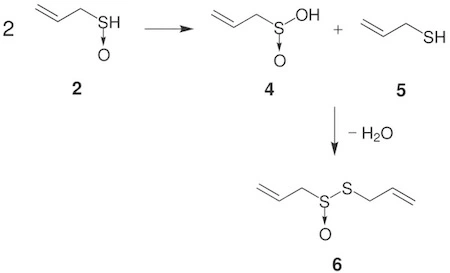
The structure of allicin was determined by Stoll and Seebeck in 1948. The substrates alliin and other sulfoxides are converted to sulfenic acids and then to thiosulfinates rapidly at room temperature. Alliinase hydrolyses alliin and other S-alkyl-L-cysteine sulfoxides to produce sulfenic acid. Hydrolysis is the reaction in which water molecule breaks (lyses) the other reactant. Then, the condensation of two molecules of allyl sulfenic acid produces one molecule of allicin. Therefore, it contains two groups of allyl or alkenes as hydrocarbon chains. Alkenes are a class of hydrocarbon compounds that contain one or more pairs of carbon atoms with double bonds between them. There are also two atoms of sulfur which are connected, forming a disulfide linkage. One of the sulfur atoms is a lone pair donor to an oxygen atom. The oxygen atom in this instance has a formal negative charge, while the sulfur atom has a formal positive charge.
Properties of Allicin
Allicin is a water-soluble and volatile organic compound. As explained earlier, allicin is a product of defense mechanisms. Such chemicals secreted by an organism to prevent a predator attack are called defensive chemicals. They have toxic effects that either repel, harm, or deter predators or competitors, or attack prey. They attack the sense of smell (olfactory systems), sense of taste and flavor (gustatory), or sense of touch (tactile sensory systems). Certain compounds also warn of the presence of a predator. These are also included in the category of defensive chemical species. Defensive secretions can be transmitted via air, water, or substrate. They can be released externally, as the dissipated VOCs are by garlic, or they can be injected directly inside the bodies of the target species when the latter tries to consume the host. Chemical defense is used by all different kinds of living organisms, from bacteria to plants and animals. It is an effective strategy to reduce the risk of being predation. For example, some plants release chemicals as a defense against animals to avoid being eaten. Antibiotics are also the result of a defensive strategy by microbes.
The Allium genus contains cysteine sulfoxides and ?-glutamyl cysteines, which derive compounds like allicin with strong lachrymatory properties. These properties sensitize the lachrymatory glands in the eyes, nose, and mouth, causing the release of secretions. Allicin can oxidize amino acid residues such as thiols in cells, as it is a reactive sulfur species (RSS). Allicin is a volatile organic sulfur compound (VOSC) that degrades to create more VOSCs like polysulfanes and ajoene. It is a colorless liquid at room temperature. It is easily degraded by heat and its antibacterial properties are nullified on heating it to 80° Celsius. The melting point of allicin is 25°C, and its boiling point is 248°C. It is soluble in water and miscible in organic solvents like alcohol, benzene, and ether.
To unlock this lesson you must be a Study.com Member. Create your account
Frequently Asked Questions
How do you get allicin?
When garlic is chopped or crushed, alliinase reacts with alliin, and allicin is formed. Allicin is the condensation product of sulfenic acids.
What foods have allicin?
Garlic from the genus allium has alliin. Isomers of alliin and other allyl cysteine sulfoxides are found in the family Alliaceae, which includes onions, garlic, and shallots. Some species of genus Brassica, like cauliflower and cabbage, also contain organosulfur compounds.
Like this project
Posted Jul 10, 2023
Learn about allicin. Understand what allicin is, its chemical formula, structure, properties, and how the allicin acts as such self-defense in garlic.
Likes
0
Views
26
Clients

Study.com