Scientific Editorial
Musclin: A Myokine with Skeletal Adaptation and Metabolic Implications
Regular exercise and the maintenance of daily physical activity is necessary for a higher quality and longer life. Exercise has also been used as lifestyle treatments and preventative measures for diseases like cancer cachexia and diabetes mellitus (1,2). As such, there has been an increasing interest in mechanisms that facilitate the health benefits of exercise. One
such factor is myokines which are signalling molecules released by skeletal muscles that may guide the muscles’ adaptation, repair or maintenance (3). Musclin is a novel myokine that is
induced by exercise and is critical for enhancing physical endurance (3). Musclin has a role in increasing mitochondrial biogenesis, oxidative phosphorylation potential and skeletal muscle
adaptations, and also influences insulin signalling through the calcium ion - protein kinase B - forkhead box O1 transcription factor (Ca2+-Akt-FOXO1) signalling cascade, impacting glucose metabolism (3). Further research into the mechanisms of musclin will increase the understanding of the effectiveness of exercise and also elucidate potential uses of musclin as a biomarker or therapeutic agent (1–3).
In a 2015 study, Subbotina et al. established the release of exercise-induced musclin in mice and investigated how musclin drives skeletal muscle adaptations for physical endurance (3). Using wild-type (WT) mice and mice that have had the musclin-encoding gene, Ostn, knocked out (Ostn-KO), the authors demonstrated that musclin was required to achieve higher workloads, higher velocities and longer durations during exercise through a treadmill program with progressive increases in speed and incline. Musclin was implicated in increasing mitochondrial biogenesis and oxidative phosphorylation potential by assessing mitochondrial content and muscle fibre types in electron micrographs of the tibialis anterior and measuring maximal aerobic capacity (VO2 max) through indirect calorimetry. Further myoblast cultures from the gastrocnemius indicated a synergistic relationship between musclin and atrial natriuretic peptide (ANP), which competitively bound to receptors to increase cyclic guanosine monophosphate (cGMP) – a known regulator in skeletal muscle mitochondrial biogenesis. The study also uses Western blots from sedentary and exercising WT mice to demonstrate that exercise-induced musclin increases levels of phosphorylated Akt and FOXO1, further corroborated by skeletal myoblast cultures where this relationship
occurred in the presence of Ca2+ ions and ionophores in a dose-dependent way.
Musclin related skeletal muscle adaptations and physical endurance is primarily driven by its synergistic relationship with ANP (3). Containing a region that is homologous to natriuretic peptides, musclin competes with ANP to bind to natriuretic peptide receptor 3 on myocytes, potentiating ANP’s activation of cGMP (4,5). However, Subbotina et al.’s measurements of ANP did not indicate significant differences, like other studies have (4,5), due to the limited volume of the terminal blood draw from the mice that cannot account for the high variability in circulating ANP. Although musclin is acutely associated with type IIb glycolytic muscle fibres, its ability to contribute to high cGMP levels stimulate mitochondrial biogenesis which improves the body’s oxidation capacity and VO2 max (3,6). As such, type I oxidative muscle fibres have been known to be more resistant to cancer-induced muscle atrophy than type II muscle fibres, and thus musclin infusions have been implicated in preventative measures or treatments for cancer cachexia (1,7). Musclin’s potentiation of ANP also has systematic effects of increasing cardiomyocyte contractility in the heart which mitigates the risks of heart failure in cancer cachexia patients, increasing its viability as a treatment for patients who are unable to perform aerobic exercise (1,8). Although the 2015 study mentions
musclin’s negative correlation with the Ca2+-Akt-FOXO1 signalling cascade, it does not contribute these findings to the discussion which limits its investigation on skeletal muscle adaptations (3). Since FOXO1 has been implicated in regulating insulin signalling, its negative relationship with musclin suggests increased musclin is a strong contributor to the type IIb muscle fibre’s lack of glucose uptake (9,10). As such, increased levels of musclin indicates higher insulin resistance (11). This implicates high levels of musclin in the pathogenesis of and as a potential biomarker for insulin-resistant type 2 diabetes mellitus (2,12).
Future studies on musclin should incorporate the long-term benefits and risks of musclin as a therapeutic agent. Studies indicate how musclin’s physiological role could be adapted into a treatment for cancer cachexia (1,8), but there is a lack of literature surrounding musclin treatment’s long-term impact on the body, especially if it is not induced by exercise. Although the 2015 study involves a musclin infusion that “rescues” the physical endurance of Ostn-KO mice, exogenous musclin’s impact on the cancer tumour and potential muscle atrophy should be considered (3).
Although musclin’s physiological role and influences have only been partially understood, it is clear that musclin has a vital role in exercise performance and endurance enhancements especially after aerobic exercise. Musclin’s role in glucose metabolism and its viability as a
treatment option is less understood but is a promising field of study that is worth investigating for diseases like cancer cachexia and diabetes mellitus.
Schematic
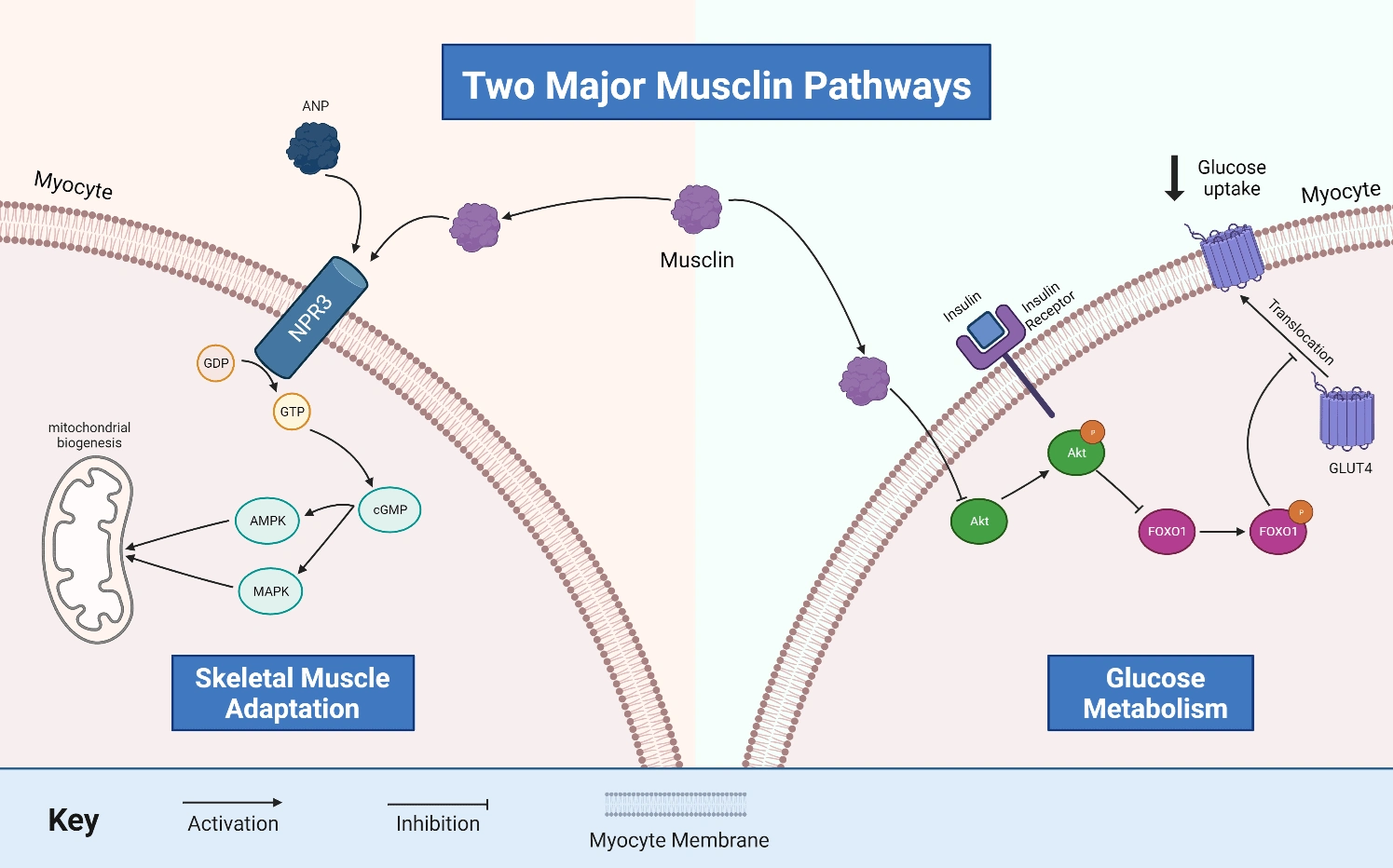
Figure 1: Two Major Musclin Pathways. The diagram outlines musclin’s pathways
that are implicated in skeletal muscle adaptation and glucose metabolism. Musclin competitively binds to the g-protein coupled receptor, NPR3 (natriuretic peptide receptor 3), against ANP (atrial natriuretic peptide) to initiate a signalling cascade that increases cGMP (cyclic guanosine monophosphate) which activates AMPK (AMP activated protein kinase) and MAPK (mitogen-activated protein kinase) to increase mitochondrial biogenesis. On the other side, musclin phosphorylates Akt (protein kinase B) in the insulin signalling pathway
which phosphorylates FOXO1 (forkhead box O1 transcription factor), which inhibits the translocation of GLUT4 (glucose transporter type 4) to the myocyte membrane, downregulating the GLUT4 receptor expression and thus decreasing glucose uptake in myocytes. GDP = guanosine diphosphate, GTP = guanosine triphosphate.
References
1. Re Cecconi AD, Forti M, Chiappa M, Zhu Z, Zingman L V., Cervo L, et al. Musclin, a
myokine induced by aerobic exercise, retards muscle atrophy during cancer
cachexia in mice. Cancers (Basel). 2019 Oct 1;11(10).
2. Chen WJ, Liu Y, Sui Y Bin, Zhang B, Zhang XH, Yin XH. Increased circulating levels
of musclin in newly diagnosed type 2 diabetic patients. Diab Vasc Dis Res. 2017
Mar 1;14(2):116–21.
3. Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SRK, Reyes S, et al. Musclin is an
activity-stimulated myokine that enhances physical endurance. Proc Natl Acad
Sci U S A. 2015 Dec 29;112(52):16042–7.
4. Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, et al. Musclin, a Novel
Skeletal Muscle-derived Secretory Factor. Journal of Biological Chemistry. 2004
May 7;279(19):19391–5.
5. Kita S, Nishizawa H, Okuno Y, Tanaka M, Yasui A, Matsuda M, et al. Competitive
binding of musclin to natriuretic peptide receptor 3 with atrial natriuretic
peptide. Journal of Endocrinology. 2009;201(2):287–95.
6. Banzet S, Koulmann N, Sanchez H, Serrurier B, Peinnequin A, Bigard AX. Musclin gene
expression is strongly related to fast-glycolytic phenotype. Biochem Biophys
Res Commun. 2007 Feb 16;353(3):713–8.
7. Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type
specificity in muscle wasting. Vol. 45, International Journal of Biochemistry
and Cell Biology. Elsevier Ltd; 2013. p. 2191–9.
8. Szaroszyk M, Kattih B, Martin-Garrido A, Trogisch FA, Dittrich GM, Grund A, et al.
Skeletal muscle derived Musclin protects the heart during pathological
overload. Nat Commun. 2022 Dec 1;13(1).
9. Liu Y, Huo X, Pang XF, Zong ZH, Meng X, Liu AG. Musclin Inhibits Insulin Activation
of Akt/Protein Kinase B in Rat Skeletal Muscle. Vol. 36, The Journal of
International Medical Research. 2008.
10. Yasui A, Nishizawa H, Okuno Y, Morita K, Kobayashi H, Kawai K, et al. Foxo1 represses
expression of musclin, a skeletal muscle-derived secretory factor. Biochem
Biophys Res Commun. 2007 Dec 14;364(2):358–65.
11. Chen WJ, Liu Y, Sui Y Bin, Yang HT, Chang JR, Tang CS, et al. Positive association
between musclin and insulin resistance in obesity: Evidence of a human study
and an animal experiment. Nutr Metab (Lond). 2017 Jul 10;14(1).
12. Yu J, Zheng J, Liu XF, Feng ZL, Zhang XP, Cao LL, et al. Exercise improved lipid
metabolism and insulin sensitivity in rats fed a high-fat diet by regulating
glucose transporter 4 (GLUT4) and musclin expression. Brazilian Journal of
Medical and Biological Research. 2016 Apr 29;49(5).
Like this project
Posted Nov 30, 2023
Developed an editorial that evaluated the reliability and applicability of a scientific paper in its field.
Likes
0
Views
12
Tags