Theory on Bypassing Heisenberg Uncertainty Principle
Imagine a single subatomic particle is isolated and shot at a constant speed, for example, the speed of light, along a straight vacuum. The speed of the object is known at any point in the vacuum, being a constant speed, and we would be able to work out the position of that object, if the measurement was also conducted at the speed of light.
Yet that would defy the basis of the Heisenberg Uncertainty Principle, which states that it is impossible to find out the position and speed of a subatomic particle simultaneously.
The Heisenberg Uncertainty Principle is often understood by physicists to be a by-product of wave- particle duality1. The position of a particle is concrete, but its speed is unknown, and vice-versa for an electromagnetic wave. However, if we have an understanding that objects act like a wave at times and a particle at other times, rather than acting as a wave and particle simultaneously, as proposed in the theory of quantum superposition regarding wave and particle duality2, then the Heisenberg Uncertainty Principle can be bypassed in this Paradox entirely.
The subatomic particle travels as a wave, but at any one moment, is a particle. This has already been somewhat established from the Copenhagen Interpretation onwards as a blur of probability3, but I would like to argue that the subatomic particle acts as a wave in every other scenario apart from a few specific instances, such as in a collision or a bond with another particle, when the particle is stationary, or when the particle is measured at one point in time, the instance present in the Paradox.
This theory would also explain other instances presented in the concept of wave-particle duality such as the photoelectric effect4, the double slit experiment5, quantum entanglement6, and countless others proposed during the first quantum revolution. The theory can also be proved mathematically as such:
Let us use the equation for finding the Reduced Planck’s constant, ħ = h/2π. Substituting h = E/f gives us E/f ÷ 2π = E/2πf, which implies that ħ = E/2πf. We can substitute this into the Heisenberg Uncertainty Principle, ΔxΔp ≥ ħ/2, to give ΔxΔp ≥ (E/2πf)÷2, or ΔxΔp ≥ E/4πf. Einstein’s energy- mass equivalence equation, E = mc2, means we can substitute E to give the final equation ΔxΔp ≥ mc2/4πf.
1 Orzel, C., & Ted-Ed. (2014, September 16). What is the Heisenberg Uncertainty Principle? - Chad Orzel. YouTube. https://www.youtube.com/watch?v=TQKELOE9eY4
2 De Ronde, C. (2016, March 19). Quantum Superpositions and the Representation of Physical Reality Beyond Measurement Outcomes and Mathematical Structures. core.ac.uk. http://philsci- archive.pitt.edu/13442/1/de_Ronde_-_Qunatum_Superpositions_-_FOS_2016.pdf
3 Carroll, S. (2019, September 9). Where Quantum Probability Comes From. Quanta Magazine. https://www.quantamagazine.org/where-quantum-probability-comes-from-20190909/
4 Arons, A. B., & Peppard, M. B. (1964, December 2). Einstein’s Proposal of the Photon Concept—a Translation of the Annalen der Physik Paper of 1905. American Journal of Physics. http://astro1.panet.utoledo.edu/~ljc/PE_eng.pdf
5 Aharonov, Y., Cohen, E., Colombo, F., Landsberger, T., Sabadini, I., Struppa, D. C., & Tollaksen, J. (2017, May 31). Finally making sense of the double-slit experiment. Proceedings of the National Academy of Sciences of the United States of America. https://www.pnas.org/content/114/25/6480
6 Nanxi Zou 2021 J. Phys.: Conf. Ser. 1827 012120
if Δx or Δp = 0 (we know the exact position or momentum of the object), then m = 0, but then, due to Einstein’s energy-mass equivalence equation, the energy of the object = 0, which we know is false, at least for the particle in the aforementioned Paradox, because objects must contain a store of intrinsic energy to move in the first place7, and even massless particles such as photons cannot achieve momentum without wavelength, derived from De-Broglie's p = h/λ equation, which cannot in turn exist with energy = 0, shown in the equation E = hc/λ.
To obtain an answer, we would have to have two masses, a mass = 0 which substitutes into ΔxΔp ≥ mc2/4πf, and a non-zero mass which substitutes into E = mc2. Quantum superposition only accommodates for a single invariant mass, hence why the object is able to exist in both states simultaneously. However, the theory I have presented accommodates for two masses, a spread-out wave-mass = 0, and a concentrated particle-mass ≠ 0. The object in wave form is spread out to an extent that mass becomes an intangible value. In particle form, however, the object is concentrated on a specific location in space, which causes mass to be a tangible value.
The question that now arises is how the theory explains other instances present in the topic of wave- particle duality. By refuting the current interpretation of the superposed wave-particle state, we are forced to find alternate explanations for experiments and ideals which supposedly justify the superposed state of particles.
There exists many, but the main ones we are required to find explanations for are four; quantum entanglement, the double-slit experiment, the photoelectric effect, and bonded electron orbit.
Double-slit
The current explanation behind the double-slit experiment is that of the causal interpretation of the pilot wave theory8, a hidden-variable theory which states that while an individual electron will travel through one particular slit, the ‘pilot wave’ which influences it will travel through both. The theory I have presented argues that the electron travels as a wave throughout the experiment, but then affirms itself to a particle on the screen at the end of the experiment. Both theories use the idea of wave interference affecting the net position of the electrons on the screen, however, the former accounts for the electron travelling across the experiment in only particle form, with the wave function accounting for placement and position, whereas the latter accounts for the electron only travelling in wave form across the experiment, with the electron converging onto a single position.
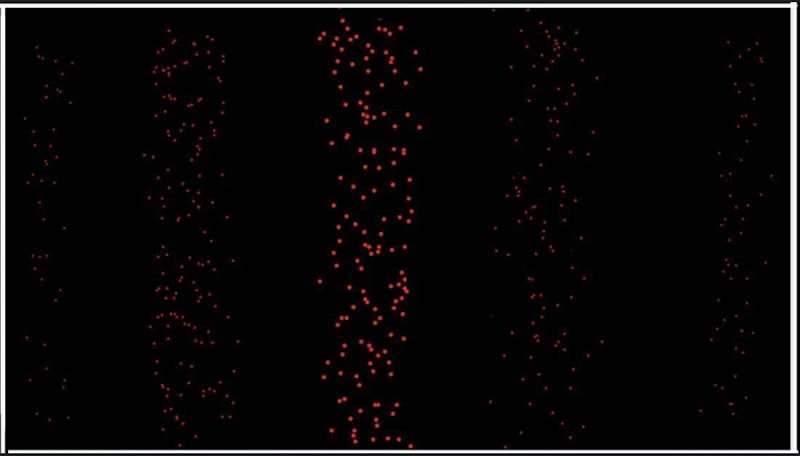
The causal interpretation of the pilot wave theory does not account for anomalies that result on the screen that go against the pattern of wave interference. Although the net positioning of electrons on the screen follows the rules of wave interference, when the experiment is carried out, the electrons seem to be in a random arrangement on the screen at the start, and only confirms to the wave interference pattern after multiple electrons are sent through the experiment. My theory suggests that the electrons travel as electromagnetic waves, subject to wave interference and wave reflection, which explains the anomalies as products of wave reflection. The resulting pattern on the screen after multiple runs can be visually represented as such:
7 Einstein, A. (1905, September 27). Does the Inertia of a Body Depend Upon Its Energy-content?
Annalen der Physik. https://www.fourmilab.ch/etexts/einstein/E_mc2/e_mc2.pdf
8 Nina Sotina 2019 J. Phys.: Conf. Ser. 1251 012046
NB: The proposed theory allows for much more flexibility regarding the number of anomalies, since the number of particles subject to wave reflection varies from experiment to experiment, thus Fig. 3 is displayed in a worst-case-scenario situation, in which a major number of electrons are affected by wave reflections. Fig. 2 is an average of multiple double-slit screens, where an anomaly is plotted based on its recurrence in the double-slit screens (A = x1 ∩ x2 ∩ x3 … ∩ xn where x = the occurrence of an anomaly and n = the number of double-slit reference screens).
The electrons of the causal interpretation are guided by the “pilot wave,” thus the particles should strictly confirm to the pattern of wave interference, however, this is often not the case. A lot of electrons confirm to the pattern of wave interference, but some deviate from the pattern of wave interference. In the theory I have proposed, the positioning of the particles remains an individual entity. Each electron follows its own path, allowing for anomalies and wave reflection against the pattern of wave interference, however, since the electron travels in wave form, the net movement still follows the pattern of wave interference, which provides a justified explanation to both the wave interference pattern and the individual anomalies.
Photoelectric effect
The photoelectric effect is also, like the Heisenberg Uncertainty Principle, understood as a by-product of wave-particle duality. Prior to Einstein’s first Annus Mirabilis paper, light was seen as only a wave, and only after 1914 was the possibility of light being a particle considered9. The aforementioned theory, however, could also accommodate for the photoelectric effect by utilising the fact that light is only required to be a particle at the point of contact. Both theories present light as exhibiting both particle and wave nature at times, however, wave-particle duality suggests that light travels as both wave and particle, whereas the theory I have presented states that light travels as a wave and confirms to a particle upon contact.
Wave-particle duality thus does not account for the requirement of wavelength for momentum, as discussed earlier. If we were to use the example presented in the Paradox, the subatomic particle would be travelling through the vacuum in both wave and particle form. However, since the particle form lacks wavelength, the particle should not be able to move from one place to another, yet that is what we observe. Both particle and wave are able to change position. The only other probable explanation is the explanation presented in the theory.
Bonded electron orbit
In a covalent bond, according to the Copenhagen Interpretation, an electron orbits in a superposed state around both atoms, such that it forms the shells of both atoms10. The theory I have presented suggests that the electron orbits around and in-between both atoms, rather than being superposed to fit the energy shells of both.
9 Franklin, A. (1997, April 1). Millikan’s Oil-Drop Experiments. Research Gate. https://www.researchgate.net/publication/226956444_Millikan’s_Oil-Drop_Experiments
10 Ted-Ed. (2014a, August 21). What can Schrödinger’s cat teach us about quantum mechanics? - Josh Samani. YouTube. https://www.youtube.com/watch?v=z1GCnycbMeA
The orbit of an electron is described as of four types, s, p, d and f orbitals, visualised respectively as such11:
11 s,p,d,f Orbitals - Chemistry | Socratic. (n.d.). Socratic.Org. Retrieved January 22, 2022, from https://socratic.org/chemistry/the-electron-configuration-of-atoms/arrangement-of-electrons-in- orbitals-spd-and-f
If we use the example of hydrogen chloride, hydrogen’s outer shell has an s-orbital (1s1), whereas chlorine’s outer shell has a p-orbital ([Ne] 3s23p5)12. This means that the electron shared between them is required to have two different energy levels. In the Copenhagen Interpretation, the electron is required to have two energy levels simultaneously, which either requires hydrogen to change to a p- orbital, which defies the exothermic nature of making the bond, or requires that chlorine change to an s-orbital, which means that hydrogen chloride takes part in electron emission, which hasn’t been detected by the compound. In the theory I have presented, the electron is individually dependent on a single atom in the covalent bond, and the force of attraction exerted by the nucleus of an atom determines the energy level, an increase in the force of attraction causing an increase in energy levels. This allows for the exothermic nature of the making of hydrogen chloride, as well as the absence of electron emission in the compound.
Entanglement
The final ideal, entanglement, is associated with superposition, as one of the conditions for entanglement to exist is that the two particles be superposed. The angular momentum of two particles can be entangled such that the angular momentum of particle A will be the opposite of particle B, if measured in the same direction13. The current understanding is that the particles are required to be superposed for the particles to be able to be able to “communicate” to inform the other particle of its angular momentum. However, the particles may be able to communicate their angular momentum without the presence of superposition.
Angular momentum can be expressed using circular polarization14. The circular polarization can be visualised in two forms; left-hand circular polarization (LHCP) and right-hand circular polarization (RHCP), shown in Fig. 8 and Fig. 9 below respectively from the point of view of the receiver:
12 Orbitals. (n.d.). Chemistry & Biochemistry - Department of Chemistry & Biochemistry. Retrieved January 22, 2022, from https://www.chem.fsu.edu/chemlab/chm1046course/orbitals.html
13 Veritasium. (2015, January 12). Quantum Entanglement & Spooky Action at a Distance. YouTube. https://www.youtube.com/watch?v=ZuvK-od647c
14 Luping, D., Zhongsheng, M., Yuquan, Z., Changjun, M., Siwei, Z., & Xiaocong, Y. (2017, January 23). Manipulating orbital angular momentum of light with tailored in-plane polarization states | Scientific Reports. Nature; Springer Nature. https://www.nature.com/articles/srep41001#Abs1
Fig. 8: LHCP from the point of view of the receiver15
16
The spin of particle A will always be the opposite of spin B, regardless of distance or time-difference at which the two are measured. However, this means that the communication is able to transcend the speed of light, which goes against Einstein’s theory of relativity.
The particles can, however, communicate indirectly without going against Einstein’s theory of relativity. Waves are best described as disturbances through matter or space17; thus the angular momentum could be communicated via the specific disturbances of particles around the wave. However, this requires that the particle be converted into the wave, rather than the wave being a separate identity, which superposition fails to offer, however, the aforementioned theory is able to offer the ability of the particle to convert into a wave.
This also applies to IBM’s quantum bit or “qubit.” The use of the quantum computer excites the qubits, which causes their spin to change rapidly multiple times, which the computer interprets as a dual state.
Conclusion
I think that the wave-particle state of matter can be better described as travelling as a wave, but converging into a particle in specific instances. This theory explains many ideals such as the double- slit experiment, the photoelectric effect, the orbit of an electron in a bond, and the theory of entanglement. The theory does not explain the Couder Experiments, albeit unnecessary due to the experiment being refuted and the results of the experiment being attributed to ‘noise, faulty
15 By Dave3457 - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=9861623
16 By Dave3457 - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=9861553
17 Learning, L. (n.d.). Waves | Boundless Physics. Lumen Learning – Simple Book Production. Retrieved January 22, 2022, from https://courses.lumenlearning.com/boundless- physics/chapter/waves/
methodology and insufficient statistics.’18, and a further test on the rate of anomalies in the double-slit experiment to confirm whether the rate of anomalies in the experiment is negligible in regards to the pilot wave theory.
The aforementioned theory may have multiple applications, one of which may be the use of the particle travelling as a wave in particle accelerators. Taking advantage of circular polarisation and entanglement so that all particles are LHCP or all particles are RHCP means that more collisions could occur. Another application of the theory may be decreasing bit flips in computers. If computer surfaces are designed to refract the wave nature of matter, or materialise the wave nature of a particle such that it remains on the surface, then the particle may not reach the motherboard, thereby significantly decreasing the rate of bit flips for the computer.
18 Wolchover, N. (2018, October 11). Famous Experiment Dooms Alternative to Quantum Weirdness. Quanta Magazine. https://www.quantamagazine.org/famous-experiment-dooms-pilot-wave- alternative-to-quantum-weirdness-20181011/
Like this project
Posted May 22, 2025
Proposed a theory to bypass Heisenberg Uncertainty Principle using wave-particle duality.
Likes
0
Views
7
Timeline
Oct 10, 2021 - Dec 24, 2021