Report on the Manufacturing Process of PI3K Inhibitors
Introduction
Phosphatidylinositol 3-kinase (PI3K) inhibitors have emerged as a promising class of drugs for the treatment of breast cancer. These inhibitors target the PI3K pathway, which is frequently dysregulated in breast cancer cells, leading to uncontrolled cell growth and survival. This report aims to provide a detailed overview of the manufacturing process of PI3K inhibitors specifically tailored for breast cancer treatment.
Synthesis of Active Pharmaceutical Ingredient (API)
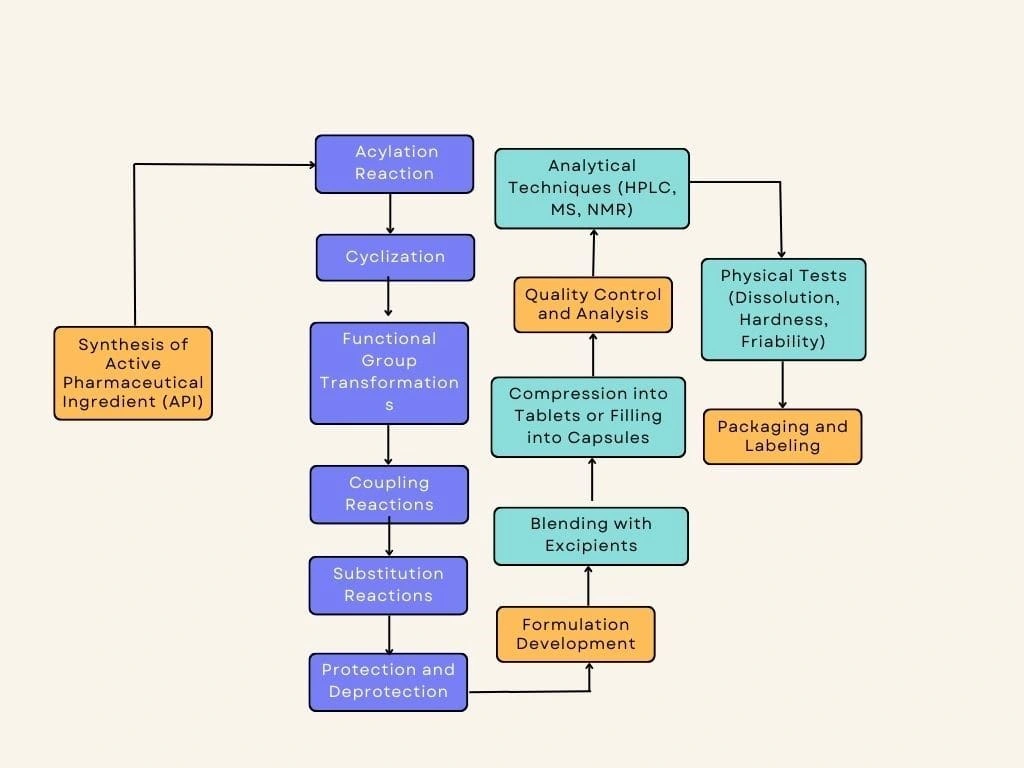
The manufacturing process commences with the synthesis of the active pharmaceutical ingredient (API), which involves a series of synthetic steps to construct the molecular scaffold of the inhibitor. Initially, an acylation reaction is employed, wherein an acyl group is introduced onto a starting material through the reaction of a carboxylic acid derivative with an appropriate nucleophile, forming an amide or ester linkage. Following this, cyclization reactions are utilized to create cyclic structures within the molecular scaffold, often achieved through intramolecular reactions such as Diels-Alder cycloaddition or ring-closing metathesis. Various functional group transformations are then executed to introduce or modify specific functional groups, including oxidation, reduction, alkylation, or deprotection reactions, tailored to optimize the compound's biological activity. Subsequent coupling reactions facilitate the joining of two fragments together, forming new carbon-carbon or carbon-heteroatom bonds, with examples including Suzuki-Miyaura, Heck, or Sonogashira couplings for constructing biaryl, alkene, or alkyne linkages, respectively. Additionally, substitution reactions are employed to introduce substituents at specific positions within the molecular scaffold, enhancing potency and selectivity. Protection strategies are integrated to selectively mask reactive functional groups during synthetic transformations, with deprotection steps removing these protecting groups once desired modifications are achieved.
Formulation Development
Upon successful synthesis of the API, the next stage involves formulation development to prepare a suitable dosage form for administration. The API undergoes blending with selected excipients such as binders, disintegrants, lubricants, and fillers to achieve a homogeneous mixture. This blend is then compressed into tablets or filled into capsules according to predetermined dosage strengths and formulation requirements. Formulation scientists meticulously optimize the composition and processing parameters to ensure uniformity and stability of the dosage form. Various physicochemical properties of the formulation, including dissolution, hardness, and friability, are evaluated to ensure the product's performance meets regulatory standards and clinical requirements.
Quality Control and Analysis
Throughout the manufacturing process, stringent quality control measures are implemented to guarantee the safety, efficacy, and consistency of the final product. Analytical techniques such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy are employed to assess the purity, identity, and stability of both the API and formulated product. Physical tests including dissolution, hardness, and friability are conducted to evaluate the dosage form's performance. These quality control measures are essential to ensure that the PI3K inhibitors meet regulatory requirements and maintain their integrity during storage and use.
Packaging and Labeling
Upon successful completion of quality control tests, the formulated product is packaged into appropriate containers such as blister packs or bottles. Packaging materials are carefully selected to provide adequate protection against environmental factors such as light, moisture, and oxygen, which could potentially degrade the drug. The product is labeled with essential information including dosage instructions, warnings, expiration date, and batch number to facilitate safe and proper use by patients and healthcare providers. Stringent adherence to packaging and labeling regulations ensures the integrity and traceability of the PI3K inhibitor products throughout their distribution and use.
Flow Diagram
The flow diagram illustrates the sequential stages of the manufacturing process, starting from the synthesis of the API through formulation development, quality control and analysis, and concluding with packaging and labeling. Each stage is represented with appropriate symbols and annotations to visually depict the flow of activities and their interconnections. The diagram provides a comprehensive overview of the manufacturing process, highlighting key steps and quality control checkpoints to ensure the production of safe and effective PI3K inhibitors for breast cancer treatment.
Like this project
Posted Jun 12, 2024
Kevin wrote a comprehensive report on the manufacturing process of PI3K Inhibitors for Breast Cancer Treatment that covered all the stages involved.
Likes
0
Views
3



