The dye SYPRO orange binds to amylin amyloid fibrils but not pr…
Abstract
Amyloid deposition underlies a broad range of diseases including multiple neurodegenerative diseases, systemic amyloidosis and type‐2 diabetes. Amyloid sensitive dyes, particularly thioflavin‐T, are widely used to detect ex‐vivo amyloid deposits, to monitor amyloid formation in vitro and to follow the kinetics of amyloid self‐assembly. We show that the dye SYPRO‐orange binds to amyloid fibrils formed by human amylin, the polypeptide responsible for islet amyloid formation in type‐2 diabetes. No fluorescence enhancement is observed in the presence of pre‐fibrillar species or in the presence of non‐amyloidogenic rat amylin. The kinetics of human amylin amyloid formation can be monitored by SYPRO‐orange fluorescence and match the time course determined with thioflavin‐T assays. Thus, SYPRO‐orange offers an alternative to thioflavin‐T assays of amylin amyloid formation. The implications for the interpretation of SYPRO‐orange‐based assays of protein stability and protein‐ligand interactions are discussed.
Keywords: SYPRO‐orange, amyloid, thioflavin‐T, islet amyloid polypeptide, amylin
Abbreviations
ANS8‐anilinonaphthalene‐1‐sulfonic acidDFSdifferential fluorescence screeningFmoc9‐fluorenylmethoxycarbonylFTIRFourier‐transform infrared spectroscopyh‐amylinhuman amylinHFIPhexafluoroisopropanolMALDImatrix assisted laser desorption/ionizationMDmolecular dynamicsPAL‐PEG5‐(4′‐Fmoc‐aminomethyl‐3′,5‐dimethoxyphenol) valeric acidr‐amylinrat amylinTEMtransmission electron microscopyTFAtrifluoroacetic acidt50time required for a human amylin to reach half maximum fluorescenceT2Dtype‐2 diabetes.
Introduction
Amyloid formation plays a significant role in a wide range of diseases including in systemic amyloidosis, multiple neurodegenerative disorders, and in type‐2 diabetes (T2D).1, 2 The universe of amyloid forming proteins is considerably broader than those implicated in disease. A large number of proteins which are not known to form amyloid in vivo can be induced to do so in vitro.3, 4 Not all amyloid formation is deleterious and functional amyloids play a beneficial role in biology.5 Amyloid is typically identified via dye binding assays; the classic reagents congo‐red and thioflavin‐S are widely used to detect amyloid deposits in tissue. The dye thioflavin‐T is the standard probe to follow amyloid formation in vitro. Binding of thioflavin‐T is believed to occur in grooves generated on the surface of amyloid fibrils by the parallel, in‐register, cross‐β structure of amyloids.6 Binding forces co‐planarity of the two rings in thioflavin‐T and relieves self‐quenching, thereby enhancing the quantum yield.7 Thioflavin‐T assays are widely applied, although they can yield false positives in amyloid inhibition assays and there are examples of proteins which form amyloid, but which do not lead to an enhancement of thioflavin‐T fluorescence in the fibril state or exhibit very weak fluorescence enhancement.8, 9 Here, we demonstrate that the commercially available dye SYPRO‐orange, which is widely used in thermal shift assays of stability, is an amyloid sensitive dye.10, 11
SYPRO‐orange is a polarity sensitive dye and was first used for post‐electrophoretic fluorescence staining of proteins.12, 13 SYPRO‐orange has been used in differential fluorescence screening (DFS) assays for high throughput thermal shift studies of protein stability and for studies of protein ligand interactions, but there are no reports of the dye binding to amyloids.14, 15, 16, 17 Here, we show that the dye selectively binds to amyloid fibrils formed by human amylin (h‐amylin, also known as islet amyloid polypeptide or IAPP), but not prefibrillar species or the non‐amyloidogenic rat amylin (r‐amylin). h‐Amylin is the protein component of the islet amyloid deposits associated with T2D.1 Islet amyloid formation by h‐amylin contributes to β‐cell death in T2D and to the failure of islet grafts.18, 19, 20
Results and Discussion
The molecular structure of SYPRO‐orange has not been reported in the literature, but it is a merocyanine‐based dye and the patent literature lists a variety of structures in this class of dye. We first conducted LC‐MS analysis of the commercially available dye to test its purity and to examine which members of this class of compounds might make up the marketed form of the dye (Supporting Information Fig. S1). Analysis of the LC trace suggests the dye is on the order of 95% pure. The observed m/z (486.3) is consistent with the structure shown in Figure 1. The figure also displays the primary sequence of human and rat amylin. The polypeptide is 37 residues in length, contains a conserved disulfide bridge between residues 2 and 7 and a conserved amidated C‐terminus.
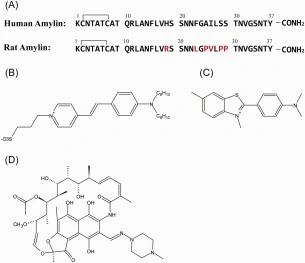
(A) Sequence of human and rat amylin. Residues in rat amylin which differ from human amylin are colored red. (B) Proposed structure of SYPRO‐orange. This structure best agrees with the patent and the LC‐MS data. (C) Structure of thioflavin‐T. (D) Structure of rifampicin.
We recorded fluorescence emission spectra of the dye in the presence of pre‐formed h‐amylin amyloid fibrils as well as prefibrillar intermediates and freshly dissolved h‐amylin (Fig. 2). A significant increase in fluorescence intensity, 6‐fold, is observed when h‐amylin amyloid fibrils are present. These studies were conducted using 16 µM h‐amylin, in monomer units. The concentration of commercially supplied SYPRO‐orange is not given in the product literature, but is supplied as a 5000x solution in pure DMSO. This solution was diluted 4000‐fold for the studies that collected the data displayed in Figure 2.
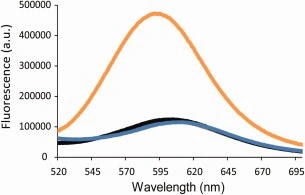
Emission spectra of SYPRO‐orange in the presence of human amylin monomers (gray), oligomers (black), fibrils (orange), and the dye alone (blue). Experiments were conducted with 16 µM human amylin, SYPRO‐orange diluted 4000‐fold from the supplied stock solution, at 25°C in 20 mM tris‐HCl at pH 7.4.
We performed additional experiments in the presence of h‐amylin fibrils (16 μm in monomer units) using a range of different dye concentrations (Supporting Information Fig. S2). A net 10,000‐fold dilution of the commercially supplied dye solution leads to higher fluorescence intensity. The most dilute sample exhibits at most approximately 30% higher fluorescence intensity than the most concentrated sample. The concentration of dye molecules present in the solution is unknown, thus it is not possible to determine the ratio of fibrils to SYPRO‐orange. The origin of the modest decrease in dye fluorescence at higher concentration is unknown, but there may be contributions from self‐quenching at higher concentrations. From a purely practical perspective, the experiment provides guidance on appropriate dilutions of the commercially supplied dye to use in h‐amylin amyloid assays.
The observation that SYPRO‐orange experiences a large increase in fluorescence intensity in the presence of h‐amylin amyloid fibrils, but not in the presence of prefibrillar species, suggests that it could be used to follow the time course of amyloid formation by h‐amylin in a fashion similar to thioflavin‐T assays. Two basic requirements need to be met if an extrinsic agent is to be used to follow amyloid formation: First, the dye should not exhibit a significant signal enhancement in the presence of monomers or pre‐fibril oligomers and second, the dye should not perturb the kinetics of amyloid formation. We conducted side by side assays of amyloid formation by h‐amylin using thioflavin‐T and SYPRO‐orange. The experiments were conducted using 16 µM h‐amylin and 32 μM thioflavin‐T or a 4000‐fold dilution of the commercially supplied solution of SYPRO‐orange. Thioflavin‐T is known to not perturb the kinetics of h‐amylin amyloid formation when added at this concentration.21 A typical thioflavin‐T time course is observed for the h‐amylin; a lag phase followed by a growth phase leading to a final plateau [Fig. 3(A)]. Similar results are observed when SYPRO‐orange is used. No detectable change in fluorescence is observed during the lag phase, but an increase in fluorescence is detected in the growth phase which displays the same time course as that detected in the thioflavin‐T assay [Fig. 3(A)]. The SYPRO‐orange fluorescence reaches a plateau at the same time as the thioflavin‐T fluorescence. T50, the time required to achieve 50% of the fluorescence change in a thioflavin‐T assay is often used to characterize the time frame of amyloid formation. The t50 for the thioflavin‐T assay is 65 ± 5.0 h and 62 ± 3.0 h for the SYPRO‐orange assays under the conditions of these experiments. To within the experimental precision, the values of T50 are identical, indicating that SYPRO‐orange does not perturb the kinetics of h‐amylin amyloid formation under these conditions. We also recorded transmission electron microscopy (TEM) of the products of the reactions. In both cases, dense mats of amyloid fibrils were observed [Fig. 3(B)]. No detectable differences were observed in the images of the SYPRO‐orange and thioflavin‐T containing samples. These experiments indicate that SYPRO‐orange can be used to monitor the kinetics of h‐amylin amyloid formation and demonstrates that the dye does not perturb the morphology of the resulting fibrils, at least at the level that can be detected by TEM.
(A) Analysis of amyloid formation by human amylin using thioflavin‐T (black) and SYPRO‐orange (orange) as the fluorescent dye. (B) TEM images of samples of human amylin amyloid fibrils formed in the presence of thioflavin‐T (black) and SYPRO‐orange (orange). Scale bars represent 100 nm. Experiments were conducted with 16 µM human amylin, 32 µM thioflavin‐T, SYPRO‐orange diluted 4000‐fold from the supplied stock solution at 25 °C in 20 mM tris‐HCl at pH 7.4.
As a further test of the specificity of the dye for amyloid, we examined the fluorescence of SYPRO‐orange in the presence of r‐amylin [Fig. 4(A)]. Rats and mice do not form islet amyloid in vivo and the rat/mouse amylin polypeptide is non‐amyloidogenic in solution although it can form low order oligomers.22 The different behavior of the hormone is due to the six substitutions in the rat sequence relative to the human sequence [Fig. 1(A)]. These include three prolines in the rat/mouse sequence and a His‐18 to Arg replacement; collectively these substitutions reduce the ability of r‐amylin to form amyloid.23, 24, 25, 26 Thus, the rat polypeptide provides a useful negative control. No change in SYPRO‐orange fluorescence is observed when r‐amylin is incubated with the dye and TEM confirms the absence of amyloid fibrils [Fig. 4(B)]. The experiment provides excellent additional evidence that SYPRO‐orange does not bind to non‐amyloidogenic forms of amylin.
(A) Analysis of amyloid formation by r‐amylin using thioflavin‐T (black) and SYPRO‐orange (orange) as the fluorescent dye. (B) TEM images of samples of human amylin amyloid fibrils formed in the presence of thioflavin‐T (black) and SYPRO‐orange (orange). Scale bars represent 100 nm. Experiments were conducted with 16 µM human amylin, 32 µM thioflavin‐T, SYPRO‐orange diluted 4000‐fold from the supplied stock solution at 25°C in 20 mM tris‐HCl at pH 7.4. Samples were collected for TEM at the end of the experiment.
Thioflavin‐T assays are also widely employed to search for inhibitors of amyloid formation, but can be compromised by inner filter effects due to the putative inhibitor and because the compound of interest may displace thioflavin‐T from the amyloid fibrils. Inner filter effects depend on the fluorescence properties of the compounds being screened. The two dyes have relatively similar excitation wavelengths, but different emission maxima. SYPRO‐orange and thioflavin‐T have excitation maxima of 490 nm and 450 nm respectively and emit at 594 nm and 485 nm respectively (Table 1). These differences indicate that SYPRO‐orange may be useful when thioflavin‐T suffers from inner filter effects. The two excitation maxima are relatively close, thus the principle advantage of using SYPRO‐orange is that it provides a different fluorescence emission wavelength to monitor and this could be useful when the absorbance spectrum of a putative inhibitor overlaps the emission of thioflavin‐T. However, the question still remains if SYPRO‐orange can be used in inhibitor assays when false negatives are a concern because the compounds being tested either displace bound thioflavin‐T from amyloid fibrils or quench the fluorescence of the bound thioflavin‐T. Consequently, we examined the effect of a compound which is known to interfere with thioflavin‐T assays. Early work, based on thioflavin‐T assays, lead to the conclusion that rifampicin is an inhibitor of amyloid formation by h‐amylin. However, subsequent studies showed that it is not and instead showed that rifampicin interferes with thioflavin‐T assays.10
Table 1
Excitation and Emission Maxima of Fluorescent Dyes Which Have Been Used to Study H‐Amylin Amyloid Formation.
FluorophoreExcitation wavelength (nm)Emission wavelength (nm)Thioflavin‐T450485SYPRO orange490594ANS370500Bis‐ANS385495Nile Red552650
We conducted side‐by‐side assays using thioflavin‐T and SYPRO‐orange. The experiments were conducted using 16 µM h‐amylin, 32 µM thioflavin‐T, or a 4000‐fold dilution of the SYPRO‐orange. A typical sigmoidal curve was observed indicating that formation of amyloid fibrils had occurred. After 90 hrs, rifampicin was then added to a final concentration of 8 µM [Fig. 5(A)]. After addition, a rapid and drastic decrease in the fluorescence of the thioflavin‐T dye was observed. A similar effect was observed with SYPRO‐orange assay [Fig. 5(A)]. We also recorded TEM images before and after the addition of the rifampicin [Fig. 5(B), 5(C)]. Dense mats of h‐amylin amyloid fibrils were evident for both samples before and after addition of rifampicin and no detectable differences in the fibril morphology were apparent after addition of rifampicin. These experiments indicate that SYPRO‐orange can be subject to the same complications as thioflavin‐T. The exact reason for the loss of SYPRO‐orange and thioflavin‐T fluorescence in the presence of rifampicin is not known. It is possible that rifampicin binds competitively with thioflavin‐T and SYPRO‐orange. If true, this would suggest that the two dyes bind to amyloid fibrils in a similar fashion. It is also possible that rifampicin quenches the fluorescence of these dyes. Irrespective of the mechanism, the key observation is that caution should be employed when conducting inhibitor assays with either thioflavin‐T, SYPRO‐orange or any other dye.
(A) Analysis of thioflavin‐T (black) and SYPRO‐orange (orange) fluorescence in the presence of human amylin and rifampicin. (B) TEM images of human amylin samples in the presence of thioflavin‐T (black) and SYPRO‐orange (orange) before the addition of rifampicin. Aliquots for TEM were removed at 90 h, denoted by the blue arrow. (C) TEM images of human amylin samples in the presence of thioflavin‐T (black) and SYPRO‐orange (orange) after the addition of rifampicin. Scale bars represent 100 nm. Aliquots for TEM were removed at 114 h denoted by the red arrow. Experiments were conducted with 16 µM human amylin, 32 µM thioflavin‐T, SYPRO‐orange diluted 4000‐fold from the supplied stock solution at 25°C in 20 mM tris‐HCl at pH 7.4. After 90 h, rifampicin was added for a final concentration of 8 µM rifampicin and 1% DMSO.
Conclusions
The data presented here demonstrates that SYPRO‐orange can bind to amylin amyloid fibrils. Its ability to bind to amylin amyloid fibrils while not binding to monomers or oligomers or non‐amyloidogenic forms of amylin indicates that it can be used as an alternative to thioflavin‐T. The fact that it does not bind to h‐amylin lag phase intermediates also provides biophysical information on the h‐amylin oligomers. SYPRO‐orange is a polarity‐sensitive dye and the lack of fluorescence during the lag phase indicates that oligomers do not have the necessary characteristics, i.e. suitable persistent hydrophobic patches, to bind to the dye. These results are consistent with previous studies performed with another polarity sensitive small molecule 8‐anilinonaphthalene‐1‐sulfonic acid (ANS).27 ANS binds to pre‐amyloid oligomers formed by a wide variety of proteins, but not to those formed by h‐amylin. ANS does however, bind to h‐amylin amyloid fibrils.28 The ability of SYPRO‐orange to bind to h‐amylin amyloid fibrils is a potential factor to consider when interpreting thermal shift assays or when using the dye to screen stability of libraries of mutant proteins. In most cases, the dye is assumed to bind to relatively unordered aggregates that can be populated upon thermal denaturation; the data presented here shows that the dye can also bind to ordered cross‐β structures. In most cases this effect should not diminish the utility of the dye in other applications, but it may impact the analysis if the structure of the unfolded aggregate is of interest.
Materials and Methods
Peptide synthesis and purification
Peptides were synthesized with a CEM microwave peptide synthesizer on a 0.10 mmol scale utilizing 9‐fluorenylmethoxycarbonyl (Fmoc) chemistry. 5‐(4′‐Fmoc‐aminomethyl‐3′,5‐dimethoxyphenol) valeric acid (PAL‐PEG) resin was used to provide an amidated C‐terminus. Fmoc‐protected pseudoproline (oxazolidine) dipeptide derivatives were utilized as previously described.26, 29 Solvents used were ACS‐grade. β‐Branched residues, the first residue attached to the resin, pseudoproline dipeptide derivatives and the residues following the pseudoproline dipeptide derivatives were double‐coupled. Peptides were cleaved from the resin via standard trifluoroacetic acid (TFA) methods. The cleaved crude peptides were dissolved into 15% (v/v) acetic acid and lyophilized. The disulfide bond was formed in 100% dimethyl sulfoxide at room temperature. Peptides were purified via reverse‐phase high‐performance liquid chromatography (RP‐HPLC) using a Higgins Analytical Proto 300 C18 preparative column (10 mm × 250 mm). The purity of the peptides was tested using analytical HPLC. The masses of the pure peptides were confirmed with MALDI time‐of‐flight mass spectrometry. h‐amylin, expected 3903.6, observed 3902.9; r‐amylin, expected 3918.0, observed 3918.4.
Sample preparation and fluorescence assays
Stock solutions were prepared by dissolving peptide into 100% hexafluoroisopropanol (HFIP) at 1.6 mM. Solutions were filtered with 0.45 μM Acrodisc syringe filters and the required amount was lyophilized overnight to remove HFIP. Dry peptide was then dissolved into tris buffer for the fluorescence assays. Fluorescence measurements were performed using a Applied Photon Technology fluorescence spectrophotometer. Emission scans were collected using an excitation wavelength of 490 nm and emission wavelengths over a range of 520–695 nm. A slit width of 15 nm was used. Oligomers and fibrils were produced by incubating human amylin. Measurements were made at several time points; immediately after dissolving the peptides; in the middle of the lag phase (as defined by thioflavin‐T assays) and after amyloid formation was complete. The kinetics of amyloid formation was monitored using thioflavin‐T or SYPRO‐orange assays conducted at 25°C. The final concentration of the thioflavin‐T assays was 16 µM peptide, 32 µM thioflavin‐T in 20 mM tris buffer at pH 7.4. The concentration of commercially supplied SYPRO‐orange is not given in the product literature, but is supplied as a 5000x solution in DMSO. Thioflavin‐T experiments were monitored using an excitation wavelength of 450 nm and an emission wavelength of 485 nm. SYPRO‐orange experiments were recorded using an excitation wavelength of 490 nm and emission wavelength of 594 nm. A slit width of 8 nm and 10 nm were used for Thioflavin‐T and SYPRO‐orange experiments respectively and a 3 mm cuvette was used. Each point was averaged over a period of 2 min.
Transmission electron microscopy (TEM)
TEM images were collected at the Life Science Microscopy Center at the State University of New York at Stony Brook. At the end of each experiment, 15 μL aliquots of the samples used for the kinetic studies were removed, blotted on a carbon‐coated 300‐mesh copper grid for 1 min and then negatively stained with saturated uranyl acetate for 1 min.
Supporting information
LC‐MS trace of the commercially supplied dye, a plot of fluorescence intensity of several concentrations of the dye, and an absorbance spectrum of the dye.
7‐25‐16 SYPRO Supporting Final.docx.
Click here for additional data file.(464K, docx)
Acknowledgments
This work was supported by the United States National Institute of Health, GM078114, to D.P.R.
References
1. Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH (1987) Amyloid fibrils in human insulinoma and islets of langerhans of the diabetic cat are derived from a neuropeptide‐like protein also present in normal islet cells. Proc Natl Acad Sci USA 84:3881–3885. [PMC free article] [PubMed] [Google Scholar]
2. Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM (1987) Purification and characterization of a peptide from amyloid‐rich pancreases of Type 2 diabetic patients. Proc Natl Acad Sci USA 84:8628–8632. [PMC free article] [PubMed] [Google Scholar]
3. Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366. [PubMed] [Google Scholar]
4. Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol 6:1054–1061. [PubMed] [Google Scholar]
5. Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855. [PMC free article] [PubMed] [Google Scholar]
6. Levine H (1993) Thioflavin‐T interaction with synthetic Alzheimers‐disease beta‐amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 2:404–410. [PMC free article] [PubMed] [Google Scholar]
7. Biancalana M, Koide S (2010) Molecular mechanism of thioflavin‐T binding to amyloid fibrils. Biochim Biophys Acta 1804:1405–1412. [PMC free article] [PubMed] [Google Scholar]
8. Wong AG, Wu C, Hannaberry E, Watson MD, Shea JE, Raleigh DP (2016) Analysis of the amyloidogenic potential of pufferfish (Takifugu rubripes) islet amyloid polypeptide highlights the limitations of thioflavin‐T assays and the difficulties in defining amyloidogenicity. Biochemistry 55:510–518. [PMC free article] [PubMed] [Google Scholar]
9. Cloe AL, Orgel JPRO, Sachleben JR, Tycko R, Meredith SC (2011) The Japanese mutant Abeta (DeltaE22‐Abeta(1‐39)) forms fibrils instantaneously, with low‐thioflavin T fluorescence: seeding of wild‐type Abeta(1‐40) into atypical fibrils by DeltaE22‐Abeta(1‐39). Biochemistry 50:2026–2039. [PMC free article] [PubMed] [Google Scholar]
10. Meng FL, Marek P, Potter KJ, Verchere CB, Raleigh DP (2008) Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril thioflavin‐T interactions: implications for mechanistic studies beta‐cell death. Biochemistry 47:6016–6024. [PubMed] [Google Scholar]
11. Hudson SA, Ecroyd H, Kee TW, Carver JA (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J 276:5960–5972. [PubMed] [Google Scholar]
12. Steinberg TH, Haugland RP, Singer VL (1996) Applications of SYPRO orange and SYPRO red protein gel stains. Anal Biochem 239:238–245. [PubMed] [Google Scholar]
13. RP Haugland, VL Singer, LJ Jones, TH Steinberg (1996) Merocyanine dye protein stains. US Patent WO 96/36882. [Google Scholar]
14. Binkowski TA, Jiang W, Roux B, Anderson WF, Joachimiak A (2014) Virtual high‐throughput ligand screening. Methods Mol Biol 1140:251–261. [PMC free article] [PubMed] [Google Scholar]
15. Lavinder JJ, Hari SB, Sullivan BJ, Magliery TJ (2009) High‐throughput thermal scanning: a general, rapid dye‐binding thermal shift screen for protein engineering. J Am Chem Soc 131:3794–3795. [PMC free article] [PubMed] [Google Scholar]
16. Lo MC, Aulabaugh A, Jin G, Cowling R, Bard J, Malamas M, Ellestad G (2004) Evaluation of fluorescence‐based thermal shift assays for hit identification in drug discovery. Anal Biochem 332:153–159. [PubMed] [Google Scholar]
17. Nashine VC, Kroetsch AM, Sahin E, Zhou R, Adams ML (2013) Orthogonal high‐throughput thermal scanning method for rank ordering protein formulations. AAPS PharmSciTech 14:1360–1366. [PMC free article] [PubMed] [Google Scholar]
18. Westermark P, Andersson A, Westermark GT (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826. [PubMed] [Google Scholar]
19. Akter R, Cao P, Noor H, Ridgway Z, Tu LH, Wang H, Wong AG, Zhang X, Abedini A, Schmidt AM, Raleigh DP (2016) Islet amyloid polypeptide: structure, function, and pathophysiology. J Diabetes Res 2016:1–18. [PMC free article] [PubMed] [Google Scholar]
20. Cao P, Raleigh DP (2013) Folding and aggregation of islet amyloid polypeptide: from physical chemistry to cell biology. Curr Opin Struct Biol 23:82–88. [PMC free article] [PubMed] [Google Scholar]
21. Tu L‐H, Raleigh DP (2013) Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry 52:333–342. [PMC free article] [PubMed] [Google Scholar]
22. Young LM, Saunders JC, Mahood RA, Revill CH, Foster RJ, Tu LH, Raleigh DP, Radford SE, Ashcroft AE (2015) Screening and classifying small‐molecule inhibitors of amyloid formation using ion mobility spectrometry‐mass spectrometry. Nat Chem 7:73–81. [PMC free article] [PubMed] [Google Scholar]
23. Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C (1990) Islet amyloid polypeptide pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA 87:5036–5040. [PMC free article] [PubMed] [Google Scholar]
24. Green J, Goldsbury C, Min T, Sunderji S, Frey P, Kistler J, Cooper G, Aebi U (2003) Full‐length rat amylin forms fibrils following substitution of single residues from human amylin. J Mol Biol 326:1147–1156. [PubMed] [Google Scholar]
25. Jha S, Snell JM, Sheftic SR, Patil SM, Daniels SB, Kolling FW, Alexandrescu AT (2014) pH dependence of amylin fibrillization. Biochemistry 53:300–310. [PubMed] [Google Scholar]
26. Abedini A, Raleigh DP (2005) Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation‐prone polypeptide. Org Lett 7:693–696. [PubMed] [Google Scholar]
27. Younan ND, Viles JH (2015) A comparison of three fluorophores for the detection of amyloid fibers and prefibrillar oligomeric assemblies. ThT (thioflavin T); ANS (1‐anilinonaphthalene‐8‐sulfonic acid); and bisANS (4,4′‐dianilino‐1,1′‐binaphthyl‐5,5′‐disulfonic acid). Biochemistry 54:4297–4306. [PubMed] [Google Scholar]
28. Mannini B, Mulvihill E, Sgromo C, Cascella R, Khodarahmi R, Ramazzotti M, Dobson CM, Cecchi C, Chiti F (2014) Toxicity of protein oligomers is rationalized by a function combining size and surface hydrophobicity. ACS Chem Biol 9:2309–2317. [PubMed] [Google Scholar]
29. Marek P, Woys AM, Sutton K, Zanni MT, Raleigh DP (2010) Efficient microwave‐assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids. Org Lett 12:4848–4851. [PMC free article] [PubMed] [Google Scholar]
Articles from Protein Science : A Publication of the Protein Society are provided here courtesy of The Protein Society
Like this project
Posted Aug 14, 2023
Amyloid deposition underlies a broad range of diseases including multiple neurodegenerative diseases, systemic amyloidosis and type‐2 diabetes. Amyloid sensiti…
Likes
0
Views
29




