QualiPilot: Auditor
Like this project
Posted May 12, 2025
Audit your quality management system documentation in minutes, not months.
Likes
0
Views
14
Timeline
May 1, 2025 - May 16, 2025
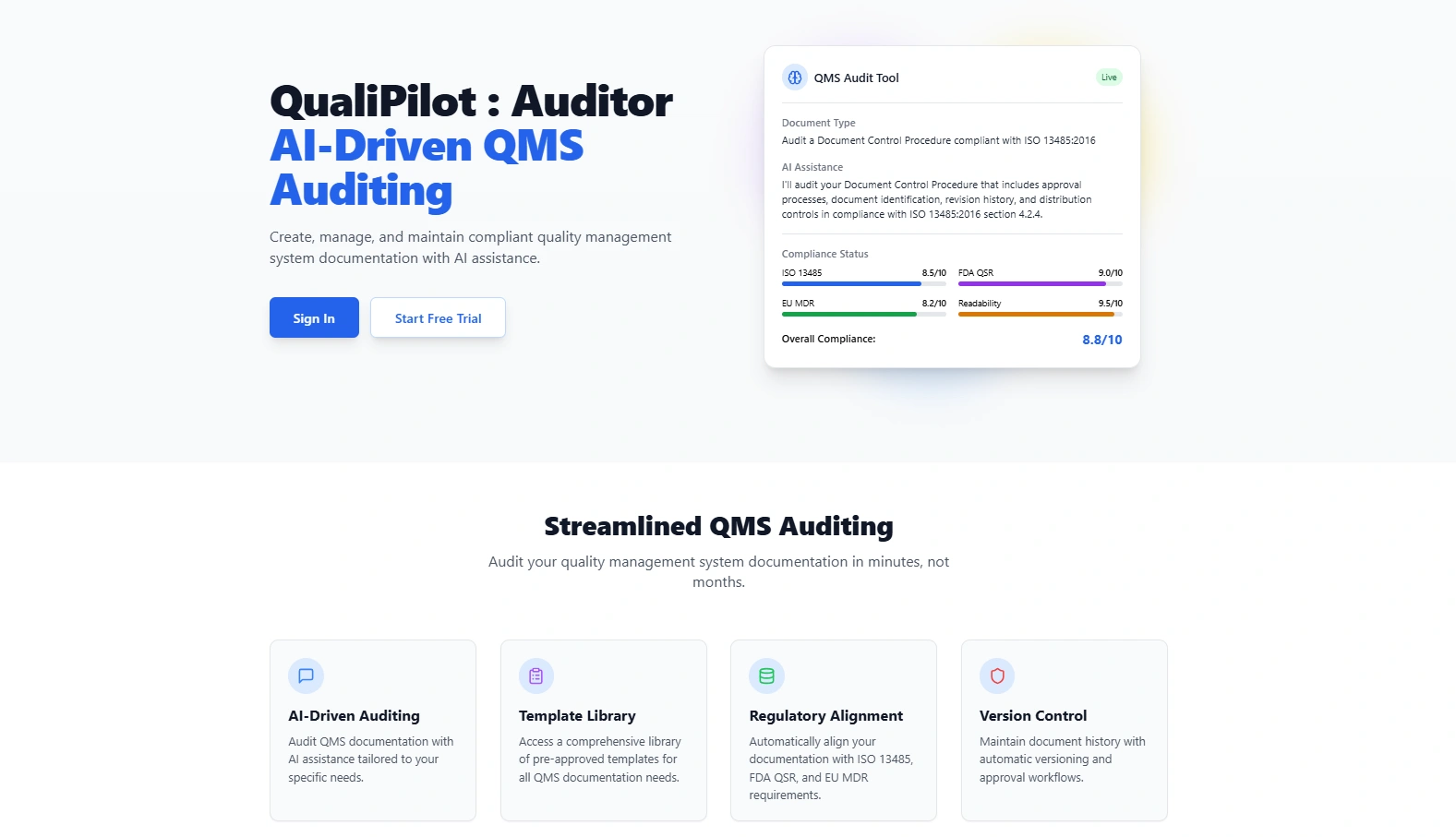
QualiPilot: Auditor is an AI-powered compliance and auditing tool designed specifically for medical device manufacturers and quality management systems. It helps organizations maintain compliance with key regulatory standards including ISO 13485:2016 and FDA 21 CFR Part 820 (Quality System Regulation).
Key features include:
Clause-Aware Auditing: The system can analyze documentation against specific regulatory clauses, identifying non-conformities and providing evidence-based recommendations.
One-Click Audit Checklists: Users can generate comprehensive audit checklists tailored to specific topics like document control, CAPA, design controls, or production processes.
AI-Powered Compliance Analysis: The compliance analyzer can evaluate specific questions against regulatory requirements, providing verdicts with supporting citations.
Auditor Chat: An AI assistant that can answer questions about regulatory requirements and provide guidance on compliance issues.
CAPA Management: A Kanban-style interface for tracking Corrective and Preventive Actions, with the ability to assign owners, set due dates, and monitor progress.
Evidence Collection: Users can upload and manage evidence files for audit findings, maintaining a complete audit trail.
Workspace Collaboration: Team-based access control with different roles (Admin, Auditor, Auditee) for collaborative auditing.
Exportable Reports: Generate PDF and CSV reports for regulatory submissions and internal documentation.